Following snap-freeze cycles from the last protocol, this next one focuses on the purification of my protein sample. I did this using immobilised metal ion affinity chromatography – as previously stated, the protein contains a His-tag, hence the means of its purification. A 1ml HiTrap purification column was used.
This protocol consisted of a number of steps:
- Wash of the column with ultra pure water
- Equilibration of the column using the same protein buffer that the protein sample was resuspended in
- Addition of protein sample in protein buffer
- Elution of the column with low concentration imidazole buffer
- Elution of the column with high concentration imidazole buffer
- Wash of the column with ultra pure water and 20% ethanol
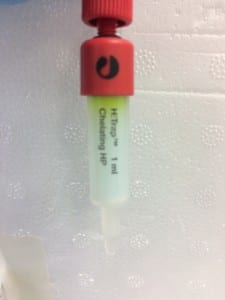
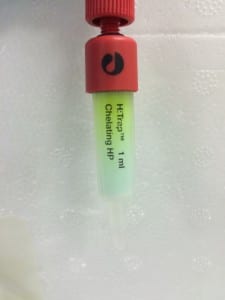
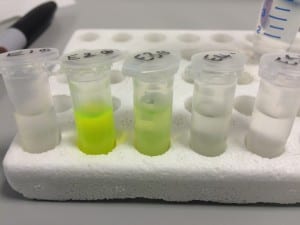
The first image above shows the column after the addition of the protein sample (again, green because it contains GFP). Notice how it forms a noticeably green band at the top of the tube, this is the region in which the protein is most highly concentrated. The second image shows the column after the first elution with low concentration imidazole buffer. For reference, imidazole is employed to elute the column because it competitively binds to the same site that Histidine attaches to. This enables the protein to move down the column once it has detached and not re-attach. The third image shows the product of elution with high concentration imidazole – 10 column volumes were run through the column and collected in separate 1 column volume aliquots, labelled here as 1-10). You can clearly see that the highest concentration was eluted in the second column volume of buffer.
Next week: SDS-PAGE to analyse the raw sample vs my newly purified sample.